Authors: Gill Morisse, Marc-Olivier Bévierre, Karl Neuberger, Jean-Baptiste Boyssou, Cyril Crawford, Sammy Hammideche
Reading time: 8 minutes
Why hasn’t the industry produced yet in a massive way these famous medical devices supposed to revolutionize medicine? On the occasion of the publication by the French HAS (Haute Autorité de Santé – French National Health Authority) of the first standard for the evaluation of connected devices embedded in AI, Quantmetry** and Cepton* are launching an ambitious cycle of articles and research to understand how these technologies will transform medicine.
Digitization, along with technological development, has been unlocking untapped potential in various fields, including healthcare. Connected Medical Devices (MD), embedded in Artificial Intelligence (AI) or not, are the most prominent example of this revolution. What opportunities can we expect from devices announced as the new technological wave in medicine? Time to dive in.
Article #1 published on Thursday, February 25 highlighted the opportunities offered by connected medical devices in the early phases of the patient care pathway (prevention and screening). In this article, we will focus on the issues at stake in the diagnosis, treatment and follow-up phases of patient care.
Patient pathway illustration:


- Diagnosis, treatment and follow-up: these steps can also benefit from key features of connected MD
A strong ally in preventing and detecting pathologies, connected MD can also prove to be very useful when it comes to treatment, although devices are less numerous mainly because of legal responsibility and technical complexity. Monitoring pathologies is key to achieve better care, while reducing the workload of medical staff and the economic burden on the healthcare system.
For example, in the field of diabetes, several solutions exist to monitor patients’ blood sugar levels continuously via their smartphone and without the need to draw blood:
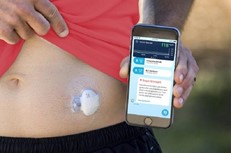
- Medtronic’s FDA-approved Guardian Connect is an FDA-approved device that integrates AI that the patient sticks on his or her abdomen and will analyze daily glucose patterns and their causes, informing patients in real time and enabling 24-hour blood glucose monitoring
- Dexcom, equipped with integrated continuous glucose monitoring (iCGM) systems for determining glucose levels, is another key player in the field of diabetology operating on the same principle.
 Dexcom solution
Dexcom solution
In the area of stroke prevention, there is Fibricheck, a cardiac rhythm monitoring device for the detection of atrial fibrillation, which has received FDA approval. To do so, the patient just needs to put his finger in front of the flash of his smartphone’s camera and the application will analyze his heart rhythm and any associated abnormalities. Following Fibricheck’s example, there is biospectal, an application that makes instant, ubiquitous and accurate remote blood pressure monitoring and management a reality. By simply placing a finger on the smartphone’s camera, anyone can turn their phone into a medical-grade blood pressure monitor with data integration into clinical care.

Fibricheck solution to prevent the onset of strokes
Some connected MDs can even go as far as administering a treatment automatically. Diabetology is, once again, a key field for these devices : Diabeloop, one of the most complex connected MD on the market, provides insulin injections monitored by numerical implants. Diabeloop’s therapeutic artificial intelligence automates insulin delivery. The DBLG1 algorithm developed by Diabeloop is hosted in a dedicated terminal that serves as a user interface with the system, associated with a continuous glucose sensor (CGM) and an insulin pump. Every five minutes, a blood glucose result is sent to the terminal via Bluetooth®. Artificial intelligence analyzes the data in real time and calculates the right dose of insulin to be administered, taking into account the patient’s personalized parameters and the information entered (meals, physical activity, etc.).
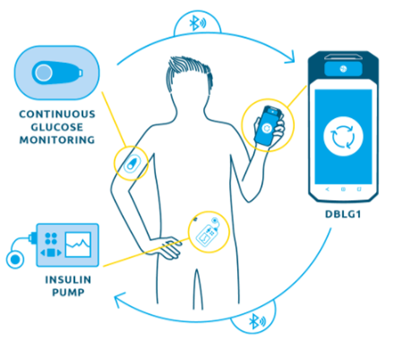
Diabeloop, solution to automate the treatment of type 1 diabetes
However, key to the patient pathway, treatment in itself, along with detection and monitoring, are not the only ways connected MD can bring opportunities to the healthcare system, and to society more globally.
By involving patients more deeply in their medication protocols or by enhancing communication with medical staff, tools such as BabyScripts can develop a healthier understanding of a science that might sound impossible to understand for many patients. This virtual maternity care platform is currently enhancing communication between obstetrics or pediatrics services and young mothers, allowing to avoid unnecessary consultations. The company offers customizable daily care for up to a year after delivery to educate and engage mothers, or an intelligent end-to-end virtual care program to manage mothers remotely. In addition, the company offers to remotely monitor the biological constants of mothers (blood pressure, weight, glycemia, mental health) by providing them with the necessary tools.
BabyScripts, solution to accompany mothers in their pregnancy
Better compliance management, helping patients stick to their treatments in the long term, might also provide a strong economic and therapeutic gain that is not often addressed in classic treatment pathways. Flaredown intends to do this, thanks to check-in with treatment tasks, daily review of health factors and automatic summaries sent on to the medical staff. Throughout the treatment process, the patient will have to answer multiple questionnaires on the application that will allow to follow as closely as possible his response to the treatment as well as his experience with the disease.
Flaredown, solution for monitoring patients’ response to treatment
Overall, connected medical devices are providing all actors along the healthcare pathway, from patients to medical staff, with crucial features that benefit economically, therapeutically, and socially. While technical complexity was a strong barrier in previous years, the industry is now developing easy-to-use, sophisticated tools, relying more and more on AI, that are unlocking personalized therapies and preventive medicine for all.
If the opportunities for the connected medical devices department are strong and growing, the number of products currently on the market is still relatively low. This can be explained by a number of barriers, particularly regulatory barriers, which the connected medical devices players have to face. These barriers will be presented in our next article.
Features of products discussed in this article:
|
|
Regulatory approval1 | Action mode2 |
Patient Involvement3 |
|||
|
FDA |
EMA | Interventional | Observational | Patient activated |
Automated |
|
|
Guardian Connect Medtronic |
✔ | ✔ | ✔ |
✔ |
||
|
Dexcom |
✔ | ✔ | ✔ |
✔ |
||
|
Fibricheck |
✔ | ✔ | ✔ | ✔ |
|
|
|
Biospectal |
✔ | ✔ |
|
|||
|
Diabeloop |
✔ | ✔ | ✔ |
✔ |
||
| Babyscripts | ✔ | ✔ | ✔ |
|
||
| Flaredown | ✔ | ✔ |
|
|||
- FDA: Food & Drug Administration; EMA: European Medicines Agency
- Interventional: effective action in the treatment; Observational: data collection process and information provision
- Patient activated: requires patient to take action; Automated: automatic and continuous operation
CEPTON and Quantmetry are launching a series of articles to understand the stakes of this transformation.
It is in this “pre-revolutionary” context that CEPTON*, a strategy consulting firm specialising in health, and Quantmetry**, a consulting firm and creator of artificial intelligence, have decided to join forces, by publishing a series of articles on this burning topic.
CEPTON and Quantmetry are convinced that these tools, which carry the seeds of a fundamental change in the way medicine is practiced, will affect healthcare professionals and regulatory authorities at the heart of their profession; it is therefore through them that this revolution will or will not take place.
The ambition of this collaboration is also to involve the actors of this this transformation: healthcare professionals, scientists, industry, regulatory institutions and, of course, patients associations.
*About Cepton:
CEPTON is a European consultancy firm specialising in the field of health. CEPTON was created in 2006 by Jean Reboullet, former Senior Partner at Roland Berger. Jean was joined a few months later by Francis Turina-Malard, former consultant at the Boston Consulting Group, then by Marc-Olivier Bévierre, who spent 15 years in the pharmaceutical industry (Novartis and Janssen) in various scientific and commercial positions, and a few years later by Philippe Cocude, with more than 25 years of experience in Healthcare consulting and general management.
CEPTON’s consulting offer covers all general management issues applied to the healthcare industry: corporate strategy, organisation, transactions and performance. CEPTON is a European-style “Strategy Boutique” that meets the expectations of executives and investors with regard to consulting: seniority, expertise, partnership and trust. We offer a rare combination of skills combining strategy consulting and transaction support.
Their long experience in leading international strategy consulting firms and in the healthcare industries has enabled CEPTON’s partners to develop a corpus of highly advanced and proven methodologies. All our consultants are specialised in the healthcare sector and are able to respond quickly and efficiently to the essential questions of the executives who place their trust in us.
**About Quantmetry:
Quantmetry is a Paris-based consulting firm that has been working with major groups for nearly 10 years to implement artificial intelligence that transforms professions.